ABOUT.
DECX Technology is the Indian distributor of Cleamix Oy (Finland) bio-decontamination equipment that generates Hydrogen Peroxide Vapour (vH2O2 / VHP). It was originally developed for the Finnish Defence force to counter CBRN warfare threats from Vx & anthrax but it is also extremely effective at eliminating Viruses, Bacteria and Harmful contaminants. It's environmentally safe because post treatment the vH2O2 safely decomposes into its constituent components of water and oxygen without leaving behind residual traces that can cause harm or irritation people or sensitive electronic equipment.Microbial decontamination
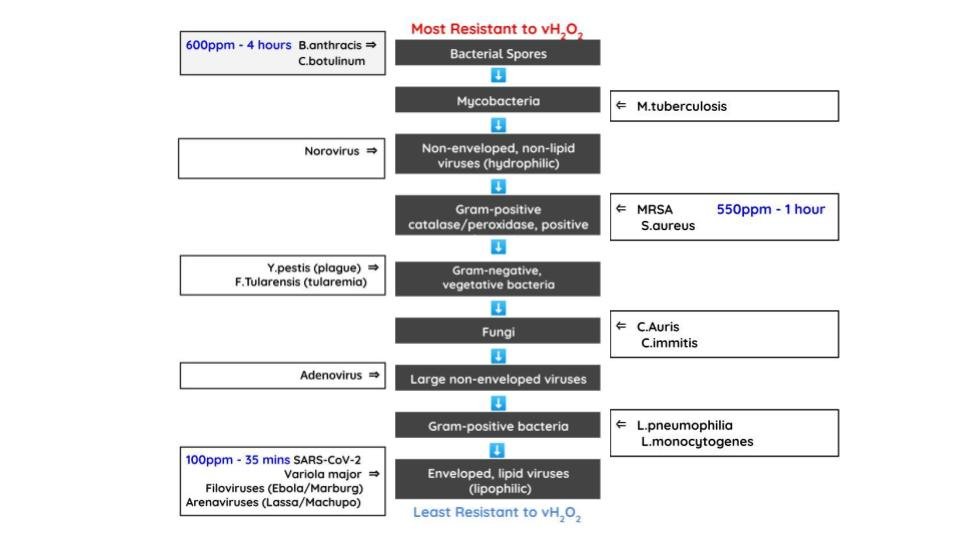
Eliminates any common biohazard with a 6-log10 reduction that includes: SARS-CoV-2 (COVID-19), C.diff, MRSA (HAI/superbugs), E. coli, staphylococcus, salmonella, norovirus (bacterial gastroenteritis) destroying the organisms and their spores present on surfaces.
RGCB Validation
Validated for use in India by the Rajiv Gandhi Centre for Biotechnology (RGCB) in Kerala at the Laboratory Medicine & Molecular Diagnostics (LMMD) under the quality system procedure of NABL ISO 15189-2012 strictly adhering to ICMR protocols.
Infection Prevention & Control
Our Vaporised Hydrogen Peroxide solution for bio-decontamination provides the highest level of microbial reduction compared to traditional cleaning methods (swipe, mop, aerosol spray) and should be a part of any Infection Prevention & Control protocol.
CBRN Defence
The same equipment can also remove contaminants from CBRN chemical warfare threats such as anthrax, VX nerve agents, it’s also NATO approved (NSN Number 6840580013819)
features
Features
Environmentally Safe
Vaporised Hydrogen Peroxide (VHP / vH2O2) is a highly versatile gaseous agent that provides excellent coverage and permeates into hard to reach areas with an extensive reach over all surfaces, 200 to 600 ppm can be generated efficiently to kill all microorganisms and their spores. It’s environmentally safe where the by-product of vH2O2 post treatment is simply water and oxygen, leaving no harmful residues that can cause irritation to people or potential damage to sensitive electronic equipment e.g medical equipment, instrumentation etc. It's extremely effective for low-temperature antimicrobial decontamination that provides > 6-log microbial reduction.
Automated & Simple Control
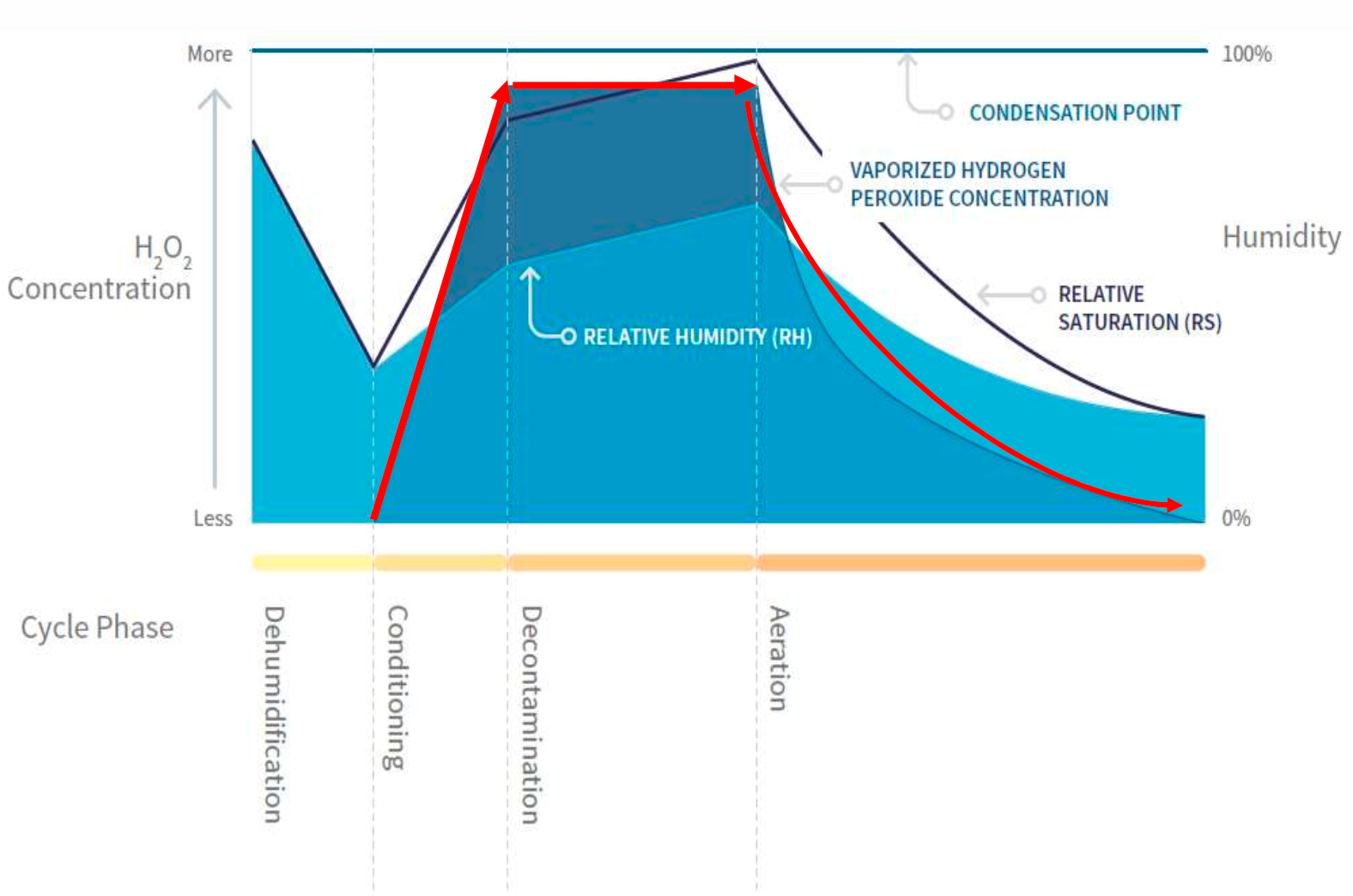
The Cleamix vH2O2 generation is accurate and repeatable through a "no-touch" automated process that is consistent unlike manually applied solutions.The sophisticated Cleamix software implements the program parameters and constantly monitors real-time sensor data (temperature, RH, RS, vH2O2 concentration in ppm), controlling the production of the hydrogen peroxide vapour levels to guarantee the ppm target level, treatment profile as well as prevent vapour saturation. VCS products support Modbus for the integration with process manufacturing equipment and logs each decontamination cycle for traceability reporting as required for example by good manufacturing practices (GMP) in pharmaceutical production.
No-touch Repeatability
vH2O2 as a gaseous agent is reliable & consistent providing excellent penetration and permeates into hard to reach locations. Accurate and repeatable through a "no-touch" automated process that is consistent every time unlike manually applied "spray and pray" solutions. You simply specify the desired VHP ppm concentration along with the treatment duration time, and the unit takes care of the rest with a fully automated treatment process of the decontamination area. The User Interface is through a touch screen on the Master Unit and remotely using computer/tablet/phone connected via WiFi or ethernet cable
Innovative
State of the art sensors for real-time monitoring of the concentration and condensation point; real-time adjustment of vH2O2 output, heating and air-drying to maintain the desired decontamination parameters. The vH2O2 produced does not leave corrosive residual traces that can damage fabric, sensitive electronic equipment or harm people, this is because we constantly monitor and control the production of the vH2O2 preventing saturation. Highly efficient vH2O2 generation with 80% output much higher compared to others, the cost of vH2O2 consumables is negligible. 1 litre of Hydrogen Peroxide liquid solution (35-50%) will deliver 335 minutes of operation at full power.
Versatile & Scaleable
From the compact and portable VCS-100 briefcase-sized unit weighing under 13kg, designed for mobility, the VC-20 version for small volume 20m2 bio-decontamination integrated in process equipment including Modbus control interface to the VCS-750 floor mounted for large volume decontamination of clean rooms etc. Up to 50 units can be networked together for the simultaneous decontamination of larger spaces / multiple rooms & compartments etc.Application Areas
Healthcare; hospitals, ambulances, A&E, ICU, wards, operating theatre, dental studio for the elimination of all viruses incl. HAI’s (Healthcare Acquired Infections), COVID-19, norovirus
Process equipment manufacture; Pharmaceutical, biosafety, pass-through chamber, clean-room, bio-containment
Hospitality; Hotel bathrooms, wash rooms, bar, restaurant, kitchen
Agri-production; food produce, food and drink packaging for the elimination of norovirus, E.coli, staphylococcus, salmonella
Government; offices, parliament, transport
Defence; CBRN (chemical warfare threats) aircraft, ships, submarines, mess halls, barracks
Facilities management; washrooms, airports, offices, salons
Transportation; taxi, bus, rail, aircraft
Just Ask
We can help you to evaluate innovative vH2O2 solutions for microbial decontamination anywhere.
ContactvH2O2 Treatment process
There are factors such as RH and Temperature that affect the required treatment cycle time but typically the total time for both the treatment and aeration process is less than 30mins for an Ambulance, 1 hour for a 50m2 hospital room and 1.5 hrs for an operating theatre. For large areas up to 50 decontamination units can be networked and synchronised together, providing a single decontamination cycle that covers the whole large area at once. Thus reducing the treatment time by avoiding a longer serialised process that would unnecessarily occupy the use of critical resources.
To ensure optimal distribution and delivery of the hydrogen peroxide vapour on all surfaces, it is recommended to use general purpose oscillating fans or blowers to circulate the vH2O2 gaseous agent throughout the treatment area. The Catalytic Converter accelerates the aeration time to reduce vH2O2 below a safe 1 ppm level from the 100-600ppm range used during various treatments. Following the aeration phase, once levels have returned below 1 ppm then it is safe to enter the treatment area.
NOTE: Above 75ppm vH2O2 is not safe for human exposure and in the unlikely event that someone should need to enter the treatment area then it should be handled by a trained operator wearing suitable PPE including a full-face respirator mask which must be worn in order to avoid an imminent risk to life and health.
Scalability Up to 50 units can be networked together for synchronised decontamination of large areas.
Treatment Scenario There are factors such as RH and temperature that affect the ramp up of vapour production and treatment cycle time but typically the total time for both the treatment and aeration process is less than 30mins for an Ambulance, 1 hour for a 50m2 hospital room and 1.5 hrs for an operating theatre.
Treatment Time from 35 minutes above 100ppm will completely destroy SARS-CoV-2
Certification Validated for use in India by the Rajiv Gandhi Centre for Biotechnology (RGCB) in Kerala at the Laboratory Medicine & Molecular Diagnostics (LMMD)
Team
Team Profile

Simon Walmsley
CxO (Chief "x" Officer)
Shyam Prasad Panicker
CxO (Chief "x" Officer)
Dr Hemalatha Beesetti
Chief Scientific Officer
We're Hiring
Bio-medical Technical SupportContact
Contact
Location:
730, 7th floor, Ecstasy Business Park, JSD Rd, Mulund (W)-Mumbai, 400080
Email:
info@decx.in
Call:
+91 98922 75838